Category: Exploring Adverse Events
Engage in meaningful discourse and gain valuable insights into the world of Adverse Events through our in-depth discussions and analyses.
-

Pregnancy and Lactation: A Case Processing Perspective
This article explores the critical safety considerations surrounding pregnancy and lactation, highlights key distinctions in case assessment. Lets explore.
-

Death vs Fatal vs Life-threatening
This article breaks down the terms such as death, fatal, and life-threatening are often used interchangeably—yet each carries a distinct regulatory meaning.
-
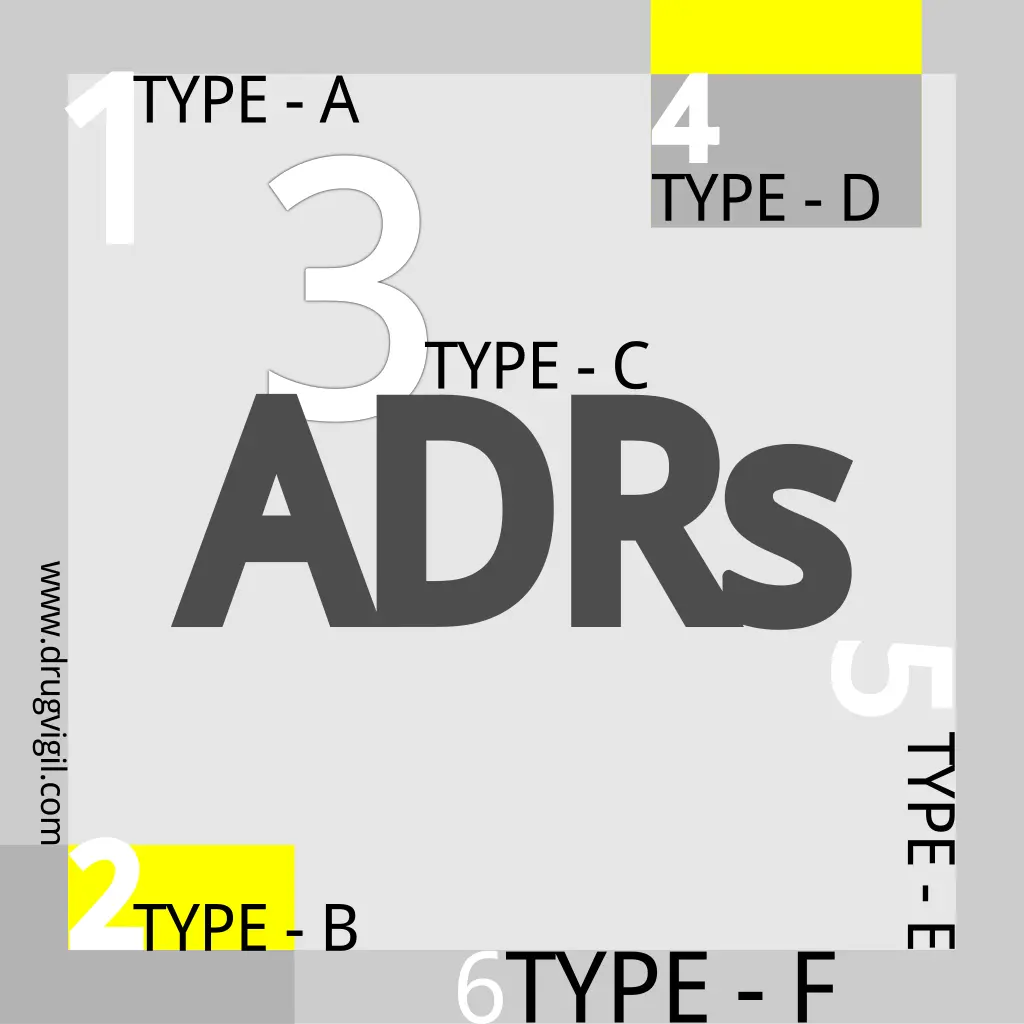
Adverse Drug Reactions: Types and Classifications Explained
In this comprehensive article, we explain the six classifieds of adverse drug reactions types, each accompanied by a brief definition.
-

Adverse Event Coding: Signs and Symptoms
This blog covers: Introduction Pharmacovigilance coding involves several complex and often confusing areas, including indications, adverse events, and current conditions. In this article, we will explore how signs, symptoms, and adverse events are identified, evaluated, and coded into a safety database, along with best practices for accurate and consistent reporting. Signs and symptoms: An overview…
-

Beyond ADRs: Reports Managed in PV
This blog covers the following: Introduction Pharmacovigilance involves much more than just managing adverse reactions or events with or without a causal relationship to a drug. Even among healthcare professionals, it’s common to refer to all safety-related reports simply as “adverse event reports.” However, pharmacovigilance encompasses a wider range of report types. In this article,…
-

Death: An Adverse Event
This blog features: Introduction This comprehensive article explores how to handle fatal cases, which are classified under the serious criteria in pharmacovigilance. It also highlights special scenarios related to managing death cases during ICSR case processing. Consider this your go-to guide to ensure you never overlook important pharmacovigilance conventions when handling such critical reports. Death…
-

Product Quality Complaints: An Adverse Event
This blog covers: Product Quality Complaint (PQC) A Product Quality Complaint (PQC) refers to any written, electronic, or oral communication alleging deficiencies related to a product’s identity, quality, durability, reliability, safety, effectiveness, or performance after its release and distribution in the market. This includes suspected medicine failures, damaged or missing products, incorrect strength or color,…
-

Disease Progression: A Special Case of Adverse Events
This article explores the concept of disease progression in pharmacovigilance, highlighting its distinction from other adverse events like Loss of Efficacy (LOE). It explains how disease progression reports can be handled.

